
In the segmentation scenario of “Life Sciences” Validation of computerized systems is a national and international regulatory requirement, which is considered a process based on the creation, development of documentation, variable analyzes and evaluation/verification of the functionalities that guarantee, through documented evidence, that the system in question is in compliance with current regulations.
Validation of computerized systems is a fundamental and mandatory process for areas with BPX/GXP impact (Impact on Quality directly or indirectly of a process or product, which may have an impact on the health of patients/clients), which must be evaluated its life cycle, that is, from its conception to its retirement, ensuring the reliability and traceability of its generated data as well as its migration when applicable.
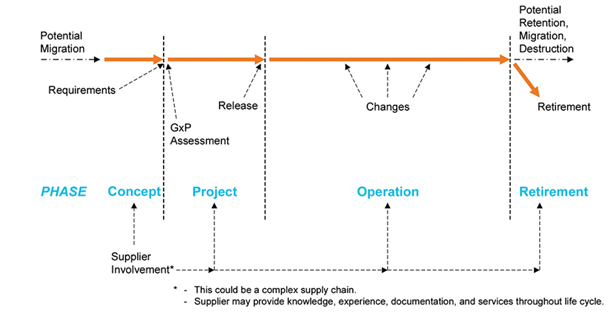
Figure 1 – Life cycle phases.
Ref.: ISPE GAMP® 5 (Second Edition) 2022.
The VSC study process must consider the development of its documentation based on the categorization dictated by GAMP5, thus making it possible to develop the ideal documentary package for the system in question.
Discover how we can help your company reach new levels of efficiency and quality!
Contact us
Service via email
Lapa,
São Paulo - SP
CEP: 05074-000

Our results come from the work of committed professionals, who seek to improve people's lives, which is why we offer Excellence in Life Sciences!
Quality Compliance Consultoria Farmacêutica Ltda CNPJ: 27.372.308/0001-14
Copyright © 2024. All Rights Reserved by VTADDONE