
Commissioning and Qualification of Equipment is a mandatory process required by legislation and regulations, aiming to ensure confidence that the equipment can be used for the intended and intended purpose.
Every process and equipment, when it comes to a regulated industry, must be tested and qualified, such a requirement is due to the fact that any failure that occurs can cause serious complications regarding effectiveness, quality and safety in the handling and storage of these products.
A failure in these industries can be considered fatal to the patient's health, therefore it is mandatory that the entire process is managed and documented from beginning to end.
It is known that within a manufacturing process, be it medicines, cosmetics, health products, veterinary products, any error can be fatal. Equipment qualification involves a series of activities in order to guarantee and document that equipment, systems and installations function as designed and meet the minimum standards required by current standards and guides.
The advantages of having qualified equipment and compliance with legislation and quality assurance are always aimed at product safety, and with this, equipment qualification also guarantees benefits for companies that are responsible for the process, namely:
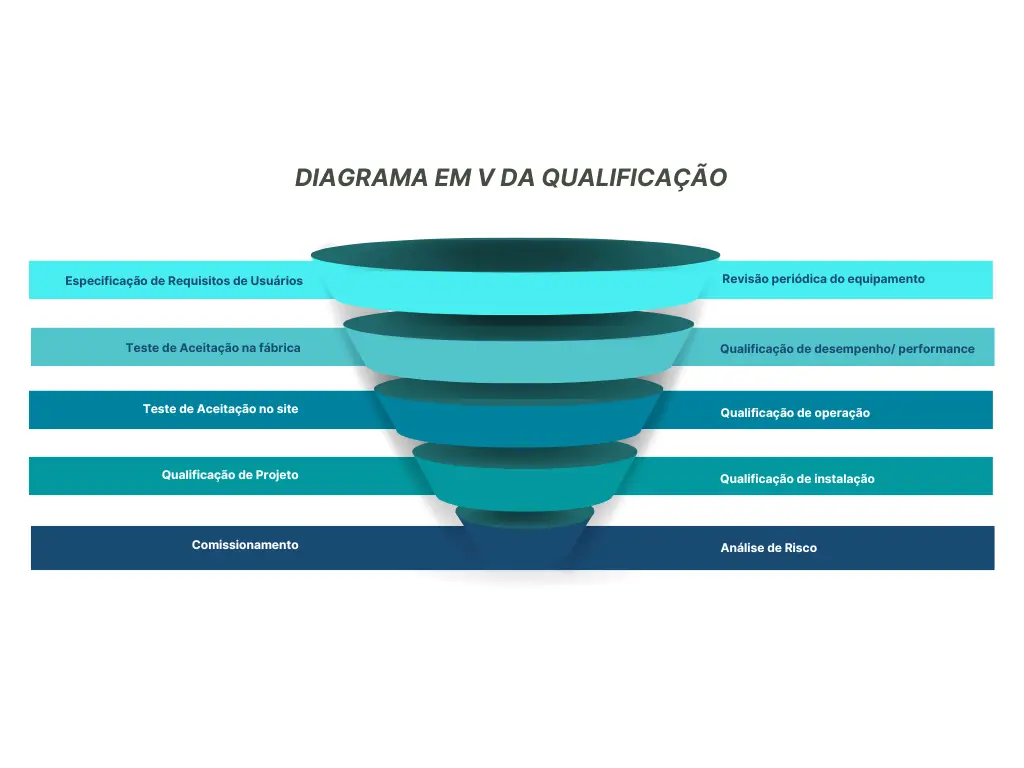
With this we can name all the phases and documentary life cycle of the equipment, namely:


Discover how we can help your company reach new levels of efficiency and quality!
Contact us
Service via email
Lapa,
São Paulo - SP
CEP: 05074-000

Our results come from the work of committed professionals, who seek to improve people's lives, which is why we offer Excellence in Life Sciences!
Quality Compliance Consultoria Farmacêutica Ltda CNPJ: 27.372.308/0001-14
Copyright © 2024. All Rights Reserved by VTADDONE