
According to IN 138, chapter 1, session lll, art. 3rd item XXIX – process validation is: “documented evidence that a process, operated within pre-established parameters, can perform its functions effectively and reproductively for the production of a medicine within its pre-established specifications and quality attributes. settled down."
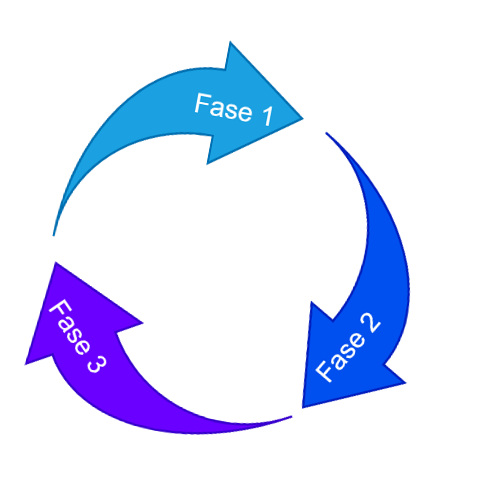
Process validation is divided into 3 distinct validations:
In order for validation to be carried out, the product development process must be robust and successful, in order to guarantee the effectiveness and reproducibility of the process under study. Therefore, process validation must consider the following phases:
Discover how we can help your company reach new levels of efficiency and quality!
Contact us
Service via email
Lapa,
São Paulo - SP
CEP: 05074-000

Our results come from the work of committed professionals, who seek to improve people's lives, which is why we offer Excellence in Life Sciences!
Quality Compliance Consultoria Farmacêutica Ltda CNPJ: 27.372.308/0001-14
Copyright © 2024. All Rights Reserved by VTADDONE